Abstract
Background: With new standard-of-care and novel treatment options available in MM, outcomes have improved; however, patients (pts) eventually become refractory to multiple drug classes (Gandhi et al. Leukemia. 2019;33:2266). Melphalan flufenamide (melflufen) is a first-in-class peptide-drug conjugate (PDC) that targets aminopeptidases and thereby rapidly releases alkylating agents inside tumor cells. In the United States, melflufen + dex received accelerated approval in RRMM based on results from the phase 2 HORIZON study (Richardson et al. J Clin Oncol. 2021;39:757). In the phase 3, randomized, OCEAN study (NCT03151811), melflufen + dex significantly reduced the risk of disease progression vs pom + dex (progression-free survival [PFS]: hazard ratio [HR], 0.79 [95% CI, 0.64-0.98; P=0.031]) in RRMM (Oncopeptides. Press release. July 8, 2021). This subgroup analysis of OCEAN investigates the impact of prior treatments on efficacy outcomes.
Methods: Eligible pts received 2-4 prior lines of therapy (LoT) including lenalidomide (len) and a proteasome inhibitor (PI) and were refractory to len (within 18 mo of randomization) and to their last LoT. Stratified by age, number of prior LoTs, and International Staging System score, pts were randomized 1:1 to 28-d cycles of melflufen 40 mg intravenously on d1 or pom 4 mg orally (PO) daily on d1 to d21. All pts received dex 40 mg (20 mg for pts ≥75 y) PO on d1, 8, 15, and 22. Pts received therapy until disease progression or unacceptable toxicity. The primary endpoint was PFS, assessed by an Independent Review Committee per International Myeloma Working Group Uniform Response Criteria. Key secondary endpoints included overall response rate (ORR), overall survival (OS), and safety. Subgroup analyses by prior therapy received were prespecified prior to the start of the study.
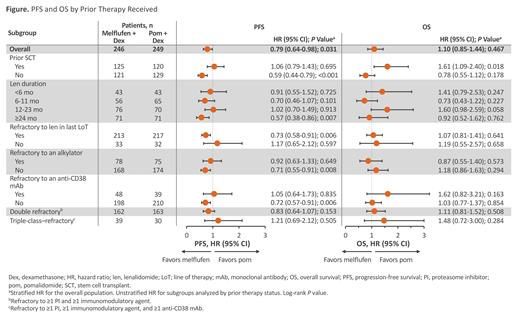
Results: At data cutoff (Feb 3, 2021), 495 pts were randomized to receive melflufen (n=246) or pom (n=249). Among these, 125 pts (51%) and 120 pts (48%) had a prior stem cell transplant (SCT), 43 pts (17%) and 43 pts (17%) were on len for <6 mo, 56 pts (23%) and 65 pts (26%) were on len for 6-11 mo, 76 pts (31%) and 70 pts (28%) were on len for 12-23 mo, 71 pts (29%) and 71 pts (29%) were on len for ≥24 mo, 213 pts (87%) and 217 pts (87%) were refractory to len in the last LoT, 78 pts (32%) and 75 pts (30%) were refractory to an alkylator, 48 pts (20%) and 39 pts (16%) were refractory to an anti-CD38 monoclonal antibody (mAb), 162 pts (66%) and 163 pts (66%) were double refractory (refractory to ≥1 PI and ≥1 immunomodulatory agent), and 39 pts (16%) and 30 pts (12%) were triple-class-refractory (refractory to ≥PI, ≥1 immunomodulatory agent, and ≥1 anti-CD38 mAb), respectively.
PFS and OS by prior therapy received are shown in the Figure. Significant PFS benefit with melflufen (vs pom) was observed in pts without a prior SCT (HR, 0.59 [95% CI, 0.44-0.79]; P<0.001), pts on len for ≥24 mo (HR, 0.57 [95% CI, 0.38-0.86]; P=0.007), pts refractory to len in last LoT (HR, 0.73 [95% CI, 0.58-0.91]; P=0.006), pts not refractory to an alkylator (HR, 0.71 [95% CI, 0.55-0.91]; P=0.008), and pts not refractory to an anti-CD38 mAb (HR, 0.72 [95% CI, 0.57-0.91]; P=0.006). Overall, the OS was 19.7 mo (95% CI, 15.4-26.0) in the melflufen group and 25.0 mo (95% CI, 18.2-32.0) in the pom group. Pts who received a prior SCT in the pom group (30.9 mo [95% CI, 20.9-36.4]) had a notably higher OS compared with the melflufen group (16.7 mo [95% CI, 14.8-not evaluable]; HR, 1.61 [95% CI, 1.09-2.40]; P=0.018), particularly in those receiving high-dose melphalan, and are being further investigated. In the melflufen vs pom arm, ORR was 42.1% vs 27.1% (P=0.013) in pts without a prior SCT, 23.2% vs 26.7% (P=0.531) in pts with a prior SCT, 35.7% vs 29.2% (P=0.449) in pts on len for 6-11 mo, 40.8% vs 25.4% (P=0.051) in pts on len for ≥24 mo, 33.8% vs 25.3% (P=0.055) in pts refractory to len in the last LoT, 36.3% vs 26.4% (P=0.049) in pts not refractory to an alkylator, 32.8% vs 26.2% (P=0.142) in pts not refractory to an anti-CD38 mAb, and 30.2% vs 28.8% (P=0.781) in pts with double refractory disease, respectively.
Conclusion: In OCEAN, melflufen + dex showed superior PFS, but not OS, compared with pom + dex. PFS benefit with melflufen was consistent across clinically relevant subgroups, primarily in pts without a prior SCT, with ongoing analyses aimed at determining factors driving this benefit.
Dimopoulos: BeiGene: Honoraria; Takeda: Honoraria; Janssen: Honoraria; BMS: Honoraria; Amgen: Honoraria. Schjesvold: Celgene/BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Schain: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Research Funding; Nordics Nanovector: Current holder of individual stocks in a privately-held company; Oncopeptides: Consultancy, Current holder of individual stocks in a privately-held company, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Adaptive Biotechnologies: Consultancy; SkyliteDX: Honoraria; Bayer: Consultancy; AbbVie: Honoraria. Mateos: Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sea-Gen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene/Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Robak: Medical University of Lodz: Current Employment; Celgene: Honoraria, Research Funding; Amgen: Honoraria; Janssen: Honoraria. Hájek: Pharma MAR: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Richardson: AbbVie: Consultancy; Janssen: Consultancy; Takeda: Consultancy, Research Funding; Sanofi: Consultancy; Celgene/BMS: Consultancy, Research Funding; Regeneron: Consultancy; GlaxoSmithKline: Consultancy; Protocol Intelligence: Consultancy; Oncopeptides: Consultancy, Research Funding; Secura Bio: Consultancy; Karyopharm: Consultancy, Research Funding; AstraZeneca: Consultancy; Jazz Pharmaceuticals: Consultancy, Research Funding. Lindberg: Affibody: Membership on an entity's Board of Directors or advisory committees; Camurus: Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Oncopeptides: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Other: Travel, Accommodations, Expenses. Thuresson: Oncopeptides AB: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Larsson: Oncopeptides AB: Current Employment; Alnylam Pharmaceuticals Inc.: Current holder of individual stocks in a privately-held company, Ended employment in the past 24 months. Sonneveld: Amgen: Consultancy, Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; SkylineDx: Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding.
Yes, this is a subgroup analysis of a phase 3 investigational study of melflufen in patients with RRMM refractory to lenalidomid.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal